
Chemistry, 09.11.2019 05:31 brooklynpage3930
The heats of combustion of ethane (c2h6) and butane (c4h10) are 52 kj/g and 49 kj/g, respectively. we need to produce 1.000 x 103 kj heat by burning one of the fuels. which fuel will emit the least amount of co2? 1. calculate the number of grams needed of each fuel: 2. calculate the number of moles of each fuel: 3. write down the balanced chemical equation for the combustion of the fuels: 4. calculate the number of moles of co2 produced by burning each fuel to produce 1.000 x 103 kj. which fuel will emit the least amount of co2?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:00
When a comet collides with earth, it adds material to our planet and causes great damage. therefore, a collision like this is a a. destructive force b. constructive force c. geologic process and event d. constructive and destructive force
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2


Chemistry, 23.06.2019 05:30
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table? atom p has an estimated zeff of 7 and is therefore to the left of atom q, which has a zeff of 6. atom p has an estimated zeff of 7 and is therefore to the right of atom q, which has a zeff of 6. atom p has an estimated zeff of 5 and is therefore below atom q, which has a zeff of 4. atom p has an estimated zeff of 5 and is therefore above atom q, which has a zeff of 4.
Answers: 3
You know the right answer?
The heats of combustion of ethane (c2h6) and butane (c4h10) are 52 kj/g and 49 kj/g, respectively. w...
Questions



Mathematics, 24.09.2019 13:30

History, 24.09.2019 13:30


Mathematics, 24.09.2019 13:30



History, 24.09.2019 13:30

Biology, 24.09.2019 13:30



Mathematics, 24.09.2019 13:30


Mathematics, 24.09.2019 13:30

Mathematics, 24.09.2019 13:30


Mathematics, 24.09.2019 13:30

Chemistry, 24.09.2019 13:30

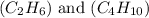 are 19.23 g and 20.41 g respectively.
are 19.23 g and 20.41 g respectively.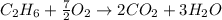
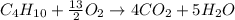
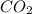 produced by burning each fuel is 1.28 mole and 1.41 mole respectively.
produced by burning each fuel is 1.28 mole and 1.41 mole respectively.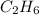
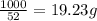
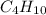 = 1 g
= 1 g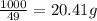
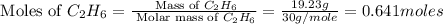
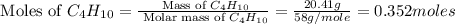
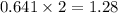 moles of
moles of 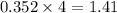 moles of
moles of 

