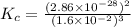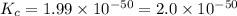Chemistry, 24.09.2019 13:30 genyjoannerubiera
At 298 k, the equilibrium concentration of o2 is 1.6 x 10-2 m, and the equilibrium concentration of o3 is 2.86 x 10-28 m. what is the equilibrium constant of the reaction at this temperature?
a. 2.0*10∧-50
b. 2.0*10∧50
c. 1.8*10∧-26
d. 1.8*10∧26


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
At 298 k, the equilibrium concentration of o2 is 1.6 x 10-2 m, and the equilibrium concentration of...
Questions

Social Studies, 20.07.2019 21:30





History, 20.07.2019 21:30

Arts, 20.07.2019 21:30



Mathematics, 20.07.2019 21:30


Mathematics, 20.07.2019 21:30








Mathematics, 20.07.2019 21:30

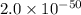
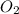 =
= 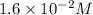
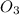 =
= 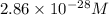
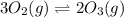
![K_c=\frac{[O_3]^2}{[O_2]^3}](/tpl/images/0258/2319/13f9f.png)