
The overall kf for the complex ion ag(nh3)2+ is 1.7 x 107. the ksp for agi is 8.5 x 10-17. what is the molar solubility of agi in a solution that is 2.0 m in nh3? the overall kf for the complex ion ag(nh3)2+ is 1.7 x 107. the ksp for agi is 8.5 x 10-17. what is the molar solubility of agi in a solution that is 2.0 m in nh3? 1.3 x 10-3 8.4 x 10-5 5.8 x 10-12 1.5 x 10-9 7.6 x 10-5

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
The overall kf for the complex ion ag(nh3)2+ is 1.7 x 107. the ksp for agi is 8.5 x 10-17. what is t...
Questions



English, 01.10.2019 01:00






Computers and Technology, 01.10.2019 01:00



Social Studies, 01.10.2019 01:00

Mathematics, 01.10.2019 01:00


Physics, 01.10.2019 01:00

Biology, 01.10.2019 01:00

Chemistry, 01.10.2019 01:00


History, 01.10.2019 01:00

Mathematics, 01.10.2019 01:00

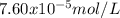
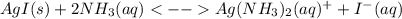
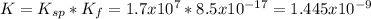
![K=\frac{[Ag(NH_3)_2^+][I^-]}{[NH_3]^2} \\K=\frac{x^2}{(2.0-2x)^2}\\\sqrt{K}= \sqrt{\frac{x^2}{(2.0-2x)^2}}\\2.0\sqrt{K}-2\sqrt{K}x-x=0\\7.60x10^{-5}-7.60x10^{-5}x-x=0\\x=\frac{7.60x10^{-5}}{1} =7.60x10^{-5}mol/L](/tpl/images/0367/6284/ce7cd.png)


