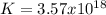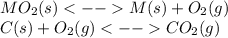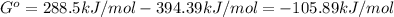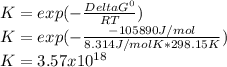Chemistry, 10.11.2019 07:31 lovelyheart5337
When the oxide of generic metal m is heated at 25c, only a negligible amount of m is produced. mo2(s) < > m(s)+o2(g) delta g = 288.5 kj/mol
1.) when the reaction is coupled to the conversion of graphite to carbon dioxide, it becomes spontaneous. what is the chemical equation of this coupled process? show that the reaction is in equilibrium, include physical states, and represent graphite as c(s)
2.) what is the thermodynamic equilibrium constant for the coupled reaction? k =

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
When the oxide of generic metal m is heated at 25c, only a negligible amount of m is produced. mo2(s...
Questions









History, 14.12.2019 03:31



History, 14.12.2019 03:31


Mathematics, 14.12.2019 03:31

Mathematics, 14.12.2019 03:31


English, 14.12.2019 03:31