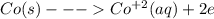Chemistry, 13.11.2019 01:31 michaelswagout
The half-cell is a chamber in the voltaic cell where one half-cell is the site of the oxidation reaction and the other half-cell is the site of the reduction reaction.
type the half-cell reaction that takes place at the anode for the cobalt-silver voltaic cell. indicate the physical states using the abbreviation (s), (l), or (g) for solid, liquid, or gas, respectively. use (aq) for an aqueous solution. do not forget to add electrons in your reaction.
co^2+ (aq) + e^- --> co was incorrect

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1


Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
The half-cell is a chamber in the voltaic cell where one half-cell is the site of the oxidation reac...
Questions












Computers and Technology, 06.08.2019 05:10