
Asheet of steel 2.5-mm thick has nitrogen atmospheres on both sides at 900°c and is permitted to achieve a steady-state diffusion condition. the diffusion coefficient for nitrogen in steel at this temperature is 1.85 × 10–10 m2/s, and the diffusion flux is found to be 1.0 × 10–7 kg/m2.s. also, it is known that the concentration of nitrogen in the steel at the high-pressure surface is 2 kg/m3. how far into the sheet from this high-pressure side will the concentration be 0.5 kg/m3

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 23.06.2019 07:30
Achemist at a pharmaceutical company is measuring equilibrium constants for reactions in which drug candidate molecules bind to a protein involved in cancer. the drug molecules bind the protein in a 1: 1 ratio to form a drug-protein complex. the protein concentration in aqueous solution at 25 ˚c is 1.74 x10-6 m . drug a is introduced into the protein solution at an initial concentration of 2.00 x10-6m. drug b is introduced into a separate, identical protein solution at an initial concentration of 2.00 x10-6m. at equilibrium, the drug a-protein solution has an a-protein complex concentration of 1.00 x10-6m, and the drug b solution has a b-protein complex concentration of 1.40 x10-6m.a. calculate the kc value for the a-protein binding reaction.b. calculate the kc value for the b-protein binding reaction.c. assuming that the drug that binds more strongly will be more effective, which drug is the better choice for further research?
Answers: 1
You know the right answer?
Asheet of steel 2.5-mm thick has nitrogen atmospheres on both sides at 900°c and is permitted to ach...
Questions




History, 20.09.2020 09:01

English, 20.09.2020 09:01




Chemistry, 20.09.2020 09:01

Mathematics, 20.09.2020 09:01





Mathematics, 20.09.2020 09:01


Mathematics, 20.09.2020 09:01


History, 20.09.2020 09:01

Arts, 20.09.2020 09:01

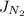 ) and the diffusion coefficient of
) and the diffusion coefficient of 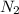 (
(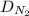 ) for the linear concentration profile at the steady state is as follows.
) for the linear concentration profile at the steady state is as follows.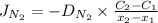
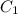 and
and 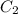 are the concentrations of
are the concentrations of 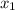 and
and 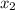 are the distances corresponding to distances on the sheet.
are the distances corresponding to distances on the sheet.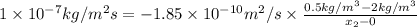
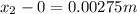
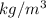 .
.

