
Chemistry, 15.11.2019 04:31 hannahgracew12
Consider the equilibrium reaction below. at high concentrations of ha the solution will be yellow; but at low concentrations of ha the solution will be red. initially the equilibrium mixture below is orange (combination of yellow & red). what color is expected after adding some colorless a–(aq) to the equilibrium solution? a-(aq) + h2o(ℓ) ⇋ ha(aq) + oh-(aq)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1


Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
Consider the equilibrium reaction below. at high concentrations of ha the solution will be yellow;...
Questions

Mathematics, 12.04.2020 20:13

Mathematics, 12.04.2020 20:13

Chemistry, 12.04.2020 20:14

Mathematics, 12.04.2020 20:14

Mathematics, 12.04.2020 20:24



Mathematics, 12.04.2020 20:24



Mathematics, 12.04.2020 20:24

Mathematics, 12.04.2020 20:25


Biology, 12.04.2020 20:25

Health, 12.04.2020 20:25

Mathematics, 12.04.2020 20:25


Mathematics, 12.04.2020 20:25


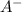 is added into the solution then it means that we are increasing the amount of reactant.
is added into the solution then it means that we are increasing the amount of reactant. 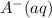 to the equilibrium solution.
to the equilibrium solution.


