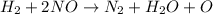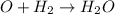Chemistry, 16.11.2019 06:31 arodriguez395
2h2(g) + 2no(g) → n2(g) + 2h2o(g) is rate=k[h2][no]2 (at least at low concentrations of h2). a possible mechanism for this reaction with two steps and an oxygen atom intermediate might take the form: h2 + 2no → n2 + x + o (step 1; k1) o + y → z (step 2; k2)
a. identify the chemical species x, y, and z in the mechanism.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1


Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3
You know the right answer?
2h2(g) + 2no(g) → n2(g) + 2h2o(g) is rate=k[h2][no]2 (at least at low concentrations of h2). a possi...
Questions


Advanced Placement (AP), 28.07.2019 06:00







English, 28.07.2019 06:00


Biology, 28.07.2019 06:00



Health, 28.07.2019 06:00





Biology, 28.07.2019 06:00

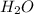 ,
, 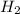 respectively.
respectively.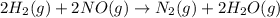
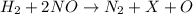
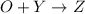
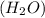 on right side of the reaction. So, the reaction 1 will be:
on right side of the reaction. So, the reaction 1 will be: