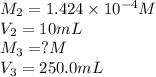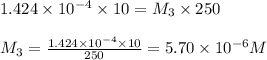Chemistry, 18.11.2019 18:31 sandeebassett3
In order to prepare very dilute solutions, a lab technician chooses to perform a series of dilutions instead of measuring a very small mass. a solution was prepared by dissolving 0.360 g of kno3 in enough water to make 500. ml of solution. a 10.0 ml sample of this solution was transferred to a 500.0-ml volumetric flask and diluted to the mark with water. then 10.0 ml of the diluted solution was transferred to a 250.0-ml flask and diluted to the mark with water. what is the final concentration of the kno3 solution? 7.91 × 10-9 m1.42 × 10-4 m5.70 × 10-6 m2.85 × 10-6 m7.12 × 10-3 m

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
In order to prepare very dilute solutions, a lab technician chooses to perform a series of dilutions...
Questions

Geography, 27.12.2019 16:31


History, 27.12.2019 16:31


Mathematics, 27.12.2019 16:31




Mathematics, 27.12.2019 16:31



History, 27.12.2019 16:31

Mathematics, 27.12.2019 16:31

History, 27.12.2019 16:31


History, 27.12.2019 16:31

Mathematics, 27.12.2019 16:31

Biology, 27.12.2019 16:31

English, 27.12.2019 16:31

Mathematics, 27.12.2019 16:31

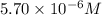
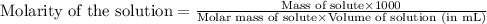
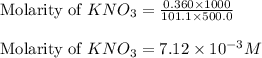
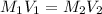 .......(1)
.......(1)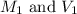 are the molarity and volume of the concentrated
are the molarity and volume of the concentrated 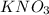 solution
solution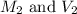 are the molarity and volume of diluted
are the molarity and volume of diluted 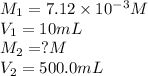
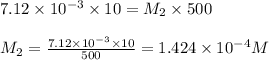
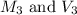 are the molarity and volume of diluted
are the molarity and volume of diluted