
Chemistry, 18.11.2019 19:31 mixedgirlmara
Consider the following equation: sio2 (s) + 3c (graphite) --> sic (s) + 2co (g) δh rxn = 624.6 kj / mol rxn. using the following standard enthalpy of formation data, calculate standard enthalpy of formation for sic (s). a. standard enthalpy of formation sio2 (s) = -910.9 kj/mol b. standard enthalpy of formation co (g) = -110.5 kj/mol

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1


Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
Consider the following equation: sio2 (s) + 3c (graphite) --> sic (s) + 2co (g) δh rxn = 624.6...
Questions

Physics, 18.03.2021 02:10

Spanish, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10

Social Studies, 18.03.2021 02:10


Computers and Technology, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10


Mathematics, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10

Biology, 18.03.2021 02:10

Spanish, 18.03.2021 02:10


Mathematics, 18.03.2021 02:10

Social Studies, 18.03.2021 02:10


Mathematics, 18.03.2021 02:10


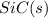 is coming out to be -65.3 kJ/mol
is coming out to be -65.3 kJ/mol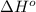
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f_{(product)}]-\sum [n\times \Delta H^o_f_{(reactant)}]](/tpl/images/0379/7554/72c39.png)
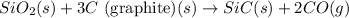
![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(SiC(s))})+(2\times \Delta H^o_f_{(CO(g))})]-[(1\times \Delta H^o_f_{(SiO_2(s))})+(3\times \Delta H^o_f_{(C(s))})]](/tpl/images/0379/7554/6a7fe.png)
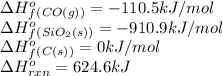
![624.6=[(1\times \Delta H^o_f_{(SiC(s))})+(2\times (-110.5))]-[(1\times (-910.9))+(3\times (0))]\\\\\Delta H^o_f_{(SiC(s))}=-65.3kJ/mol](/tpl/images/0379/7554/c8e18.png)


