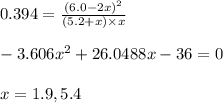Chemistry, 18.11.2019 20:31 joejoefofana
Suppose a 250 ml flask is filled with 1.3mol of o2 and 1.5 mol of no. the following reaction becomes possible: the equilibrium constant for this reaction is at the temperature of the flask. calculate the equilibrium molarity of . round your answer to two decimal places.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
You know the right answer?
Suppose a 250 ml flask is filled with 1.3mol of o2 and 1.5 mol of no. the following reaction becomes...
Questions







Health, 15.04.2020 17:31


Mathematics, 15.04.2020 17:31


History, 15.04.2020 17:31





Mathematics, 15.04.2020 17:31

Mathematics, 15.04.2020 17:31



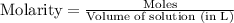
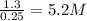
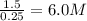
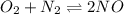
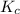 for above equation follows:
for above equation follows:![K_c=\frac{[NO]}{[O_2][N_2]}](/tpl/images/0379/8625/19733.png)
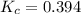 (Assuming)
(Assuming)