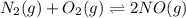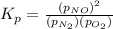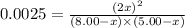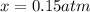Chemistry, 18.11.2019 20:31 sierravick123owr441
Nitric oxide is formed in automobile exhaust when nitrogen and oxygen in air react at high temperatures. n2(g) + o2(g) 2no(g)the equilibrium constant kp for the reaction is 0.0025 at 2127�c. if a container is charged with 8.00 atm of nitrogen and 5.00 atm of oxygen and the mixture is allowed to reach equilibrium, what will be the equilibrium partial pressure of nitrogen? a) 0.16 atm b) 0.31 atm c) 3.1 atm d) 7.7 atm e) 7.8 atm

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2



Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
Nitric oxide is formed in automobile exhaust when nitrogen and oxygen in air react at high temperatu...
Questions

History, 31.08.2019 04:10

Biology, 31.08.2019 04:10








Computers and Technology, 31.08.2019 04:10



Mathematics, 31.08.2019 04:10


Geography, 31.08.2019 04:10





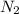 = 8.00 atm
= 8.00 atm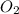 = 5.00 atm
= 5.00 atm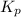 = 0.0025
= 0.0025