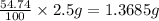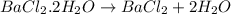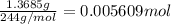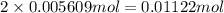Chemistry, 19.11.2019 01:31 sixtomomtermont
Astudent had a mixture of sand and barium chloride dihydrate (bacl2x2h2o). a beaker containing the mixture weighed 72.248g. when empty the beaker weighed 69.748g. the mixture was determined to contain 45.26% sand.
(a) what is the % and mass of the hydrate in the mixture?
(b) if the mixture was selectively decomposed by heating, how many grams and moles of water would be lost?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
Astudent had a mixture of sand and barium chloride dihydrate (bacl2x2h2o). a beaker containing the m...
Questions


English, 21.06.2021 14:20

English, 21.06.2021 14:20

World Languages, 21.06.2021 14:20

Biology, 21.06.2021 14:20


Mathematics, 21.06.2021 14:20

Engineering, 21.06.2021 14:20

Mathematics, 21.06.2021 14:20

Arts, 21.06.2021 14:20

Mathematics, 21.06.2021 14:20

Mathematics, 21.06.2021 14:20


Mathematics, 21.06.2021 14:20



Arts, 21.06.2021 14:20

Mathematics, 21.06.2021 14:20

Mathematics, 21.06.2021 14:20

Mathematics, 21.06.2021 14:20