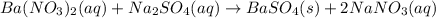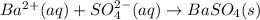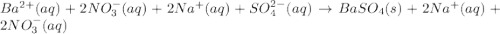Approximately 1 ml of two clear, colorless solutions-0.1 m ba(no3)2 and 0.1 m na2so4- were combined. upon mixing, a thick, milky white precipitate formed. after centrifugation, the solution above the precipitate was found to be clear and colorless. based on these observations, determine if a reaction occurred. if so, write the balanced chemical equation and net ionic equation for the reaction.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
You know the right answer?
Approximately 1 ml of two clear, colorless solutions-0.1 m ba(no3)2 and 0.1 m na2so4- were combined....
Questions



Mathematics, 09.02.2021 22:00

History, 09.02.2021 22:00

History, 09.02.2021 22:00


Chemistry, 09.02.2021 22:00



Mathematics, 09.02.2021 22:00


Social Studies, 09.02.2021 22:00


Mathematics, 09.02.2021 22:00

Physics, 09.02.2021 22:00


Biology, 09.02.2021 22:00

Social Studies, 09.02.2021 22:00

History, 09.02.2021 22:00

Advanced Placement (AP), 09.02.2021 22:00