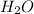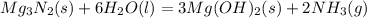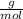Chemistry, 19.11.2019 18:31 nadiarose6345
Mg3n2(s)+6h2o(l)→3mg(oh)2(s)+2nh3(g ) when 36.0 g of h2o react, how many grams of nh3 are produced? when 36.0 g of h2o react, how many grams of nh3 are produced? 34.0 g 10.0 g 5.67 g 11.3 g 102 g

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1
You know the right answer?
Mg3n2(s)+6h2o(l)→3mg(oh)2(s)+2nh3(g ) when 36.0 g of h2o react, how many grams of nh3 are produced?...
Questions




Chemistry, 18.03.2021 02:20

Arts, 18.03.2021 02:20

Mathematics, 18.03.2021 02:20



Geography, 18.03.2021 02:20

Mathematics, 18.03.2021 02:20

Mathematics, 18.03.2021 02:20





Mathematics, 18.03.2021 02:20


Mathematics, 18.03.2021 02:20


Mathematics, 18.03.2021 02:20

 are produced from 36.0 g of
are produced from 36.0 g of