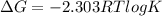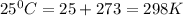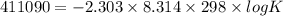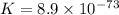Chemistry, 20.11.2019 20:31 pennyluvsu13
Use the tabulated half-cell potentials to calculate the equilibrium constant (k) for the following balanced redox reaction at 25°c. 2 al(s) + 3 mg2+(aq) → 2 al3+(aq) + 3 mg(s) a) 1.1 × 1072 b) 8.9 × 10-73 c) 1.1 × 10-72 d) 1.0 × 1024 e) 4.6 × 1031

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3
You know the right answer?
Use the tabulated half-cell potentials to calculate the equilibrium constant (k) for the following b...
Questions



English, 30.06.2019 09:00

Mathematics, 30.06.2019 09:00











Spanish, 30.06.2019 09:00

Mathematics, 30.06.2019 09:00




Mathematics, 30.06.2019 09:00

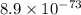
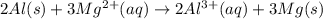
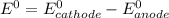
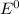 are standard reduction potentials.
are standard reduction potentials.![E^0_{[Mg^{2+}/Mg]}= -2.37V](/tpl/images/0383/2083/24fc1.png)
![E^0_{[Al^{3+}/Al]}=-1.66V](/tpl/images/0383/2083/0867c.png)
![E^0=E^0_{[Mg^{2+}/Mg]}- E^0_{[Al^{3+}/Al]}](/tpl/images/0383/2083/0b323.png)
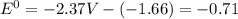

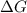 = gibbs free energy
= gibbs free energy