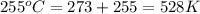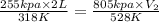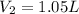Chemistry, 21.01.2020 02:31 dowlingdestiny
Agas at 255 kilopascals and 45°c has an initial volume of 2.00l. the pressure of the gas increases to 805 kilopascals as the temperature is raised to 255°c. what will be the new volume?
1.05 liters
2.28 liters
1.25 liters
0.44 liters
1.50 liters

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is common about these molecules? a.their atoms are held together by covalent bonds. b.they are all made up of the same two atoms. c.their atoms are held together by ionic bonds. d.they are all made up of oxygen atoms only.
Answers: 3

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
Agas at 255 kilopascals and 45°c has an initial volume of 2.00l. the pressure of the gas increases t...
Questions

Chemistry, 28.09.2020 16:01



Geography, 28.09.2020 16:01



Chemistry, 28.09.2020 16:01




Mathematics, 28.09.2020 16:01

English, 28.09.2020 16:01

Mathematics, 28.09.2020 16:01


Mathematics, 28.09.2020 16:01


Chemistry, 28.09.2020 16:01

History, 28.09.2020 16:01


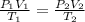
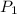 = initial pressure of gas = 255 kpa
= initial pressure of gas = 255 kpa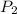 = final pressure of gas = 805 kpa
= final pressure of gas = 805 kpa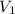 = initial volume of gas = 2 L
= initial volume of gas = 2 L
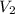 = final volume of gas = ?
= final volume of gas = ?
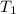 = initial temperature of gas =
= initial temperature of gas = 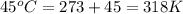
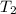 = final temperature of gas =
= final temperature of gas =