Chemistry, 23.11.2019 02:31 Lawrence101
Gas is trapped inside of a cell with volume 140 m3. the gas exerts 7600 pa of pressure against the walls of the cell. a machine then expands the cell and gives off 490 kj of heat to the gas during the process. if the internal energy changed by 140 kj, what is the final volume of the cell? assume the pressure stays constant.
a. 57 m3
b. 94 m3
c. 660 m3
d. 220 m3
e. 190 m3

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1

Chemistry, 23.06.2019 09:00
Spaghetti sauce can be high in sodium. what is a good guideline for mg of sodium per half cup serving? a. less than 1 mg b. less than 800 mg c. less than 700 mg d. less than 400 mg
Answers: 2

Chemistry, 23.06.2019 15:20
Which element below could be an isotope of berylliumsodium-10beryllium-10boron-9carbon-9
Answers: 2
You know the right answer?
Gas is trapped inside of a cell with volume 140 m3. the gas exerts 7600 pa of pressure against the w...
Questions


Mathematics, 17.09.2019 00:00


Mathematics, 17.09.2019 00:00

History, 17.09.2019 00:00

Computers and Technology, 17.09.2019 00:00


Mathematics, 17.09.2019 00:00

History, 17.09.2019 00:00



Biology, 17.09.2019 00:00


Mathematics, 17.09.2019 00:00

Biology, 17.09.2019 00:00

Business, 17.09.2019 00:00

Social Studies, 17.09.2019 00:00


Mathematics, 17.09.2019 00:00

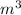 , Pressure = 7600 Pa
, Pressure = 7600 Pa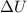 = 140 kJ
= 140 kJ 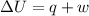
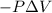
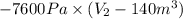
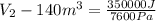
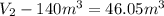
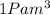 )
)