
A0.327-g sample of azulene (c10h8) is burned in a bomb calorimeter and the temperature increases from 25.20 °c to 27.60 °c. the calorimeter contains 1.17×103 g of water and the bomb has a heat capacity of 786 j/°c. based on this experiment, calculate δe for the combustion reaction per mole of azulene burned (kj/mol). c13h24o4(s) + 17 o2(g) 13 co2(g) + 12 h2o(l) e = kj/mol.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1

Chemistry, 21.06.2019 17:30
What is the percentage by mass of silicon (si) in iron aluminum silicate (fe3al2(sio4)3)?
Answers: 2

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1
You know the right answer?
A0.327-g sample of azulene (c10h8) is burned in a bomb calorimeter and the temperature increases fro...
Questions

Chemistry, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50


World Languages, 17.03.2021 23:50







Mathematics, 17.03.2021 23:50




Mathematics, 17.03.2021 23:50


Chemistry, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50


Mathematics, 17.03.2021 23:50


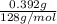



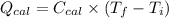
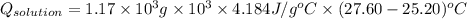
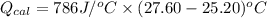
 = (11748.67 + 1886.4) J
= (11748.67 + 1886.4) J

 for the given combustion reaction per mole of azulene burned is 4452.26 kJ/mol.
for the given combustion reaction per mole of azulene burned is 4452.26 kJ/mol.

