
Chemistry, 25.11.2019 20:31 potternatalie90
What is the value of the equilibrium constant at 25 oc for the reaction between the pair: ag(s) and ni2+(aq) to give ni(s) and ag+(aq) use the reduction potential values for ag+(aq) of +0.80 v and for ni2+(aq) of -0.25 v give your answer using e-notation with no decimal places

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

You know the right answer?
What is the value of the equilibrium constant at 25 oc for the reaction between the pair: ag(s) and...
Questions



Business, 06.12.2019 20:31

Mathematics, 06.12.2019 20:31

Computers and Technology, 06.12.2019 20:31

History, 06.12.2019 20:31

Biology, 06.12.2019 20:31



Mathematics, 06.12.2019 20:31





Health, 06.12.2019 20:31

Mathematics, 06.12.2019 20:31




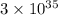
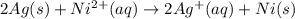
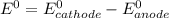
 are standard reduction potentials.
are standard reduction potentials.![E^0_{[Ag^{+}/Mg]}=+0.80V](/tpl/images/0390/1746/71d3a.png)
![E^0_{[Ni^{2+}/Ni]}=-0.25V](/tpl/images/0390/1746/2bf3e.png)
![E^0=E^0_{[Ni^{2+}/Ni]}- E^0_{[Ag^{+}/Ag]}](/tpl/images/0390/1746/494e8.png)


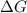 = gibbs free energy
= gibbs free energy 

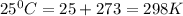

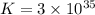
 is
is 

