
Chemistry, 26.11.2019 04:31 emiliolovato
The maximum allowable concentration of pb2+ ions in drinking water is 0.05 ppm (i. e., 0.05 g of pb2+ in 1 million grams of water). (a) what is the pb2+ concentration of an underground water supply at equilibrium with the mineral cerussite (pbco3) (ksp = 3.3 × 10−14) ? × 10 g / lenter your answer in scientific notation. (b) does this concentration exceed the allowable concentration guideline? yes no

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1


Chemistry, 23.06.2019 12:30
How does a nuclear reactor produce electricity? a. high-energy gamma rays are converted by a generator into electricity. b. the heat from the reaction turns water to steam, which runs a generator. c. the reaction produces a stream of electrons that flow through wires and into batteries. d. the heat released from the reaction is used to burn coal or gas to produce electricity. e. control rods absorb the neutrons emitted and release a stream of electrons as electricity.
Answers: 1
You know the right answer?
The maximum allowable concentration of pb2+ ions in drinking water is 0.05 ppm (i. e., 0.05 g of pb2...
Questions




Mathematics, 16.04.2020 23:23

Biology, 16.04.2020 23:23

Biology, 16.04.2020 23:23




History, 16.04.2020 23:23



History, 16.04.2020 23:23

Mathematics, 16.04.2020 23:23






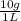 ×
×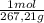 = 0,0374M
= 0,0374M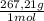 ×
×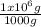 = 0,049 g of Pb²⁺ in 1 million grams of water
= 0,049 g of Pb²⁺ in 1 million grams of water

