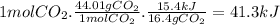Chemistry, 27.11.2019 19:31 eheheh80ii
The reaction of carbon dioxide(g) with hydrogen(g) to form carbon monoxide(g) and water(g) proceeds as follows: co2(g) + h2(g) > co(g) + h2o(g)when 16.4 grams of co2(g) react with sufficient h2(g) , 15.4 kj of energy areabsorbed .what is the value of > h for the chemical equation given? δhrxn = kj

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1

Chemistry, 23.06.2019 16:00
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a) 3.4 mol h2so4 b) 6.8 mol h2so4 c) 10.2 mol h2so4 d) 13.6 mol h2so4 a) 3.4 mol h2so4
Answers: 1
You know the right answer?
The reaction of carbon dioxide(g) with hydrogen(g) to form carbon monoxide(g) and water(g) proceeds...
Questions


Mathematics, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01

Biology, 18.10.2020 15:01

Chemistry, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01


Mathematics, 18.10.2020 15:01

Spanish, 18.10.2020 15:01


Mathematics, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01




Social Studies, 18.10.2020 15:01




Mathematics, 18.10.2020 15:01