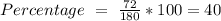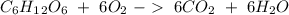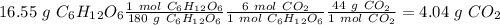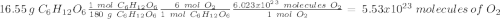Glucose (c6h12o6) is a key nutrient for generating chemical potential energy in biological systems. we were provided 16.55 g of glucose. calculate:
a) the mass percent of carbon in glucose.
b) the mass of co2 produced by the combustion of 16.55 g glucose with sufficient oxygen gas.
c) how many oxygen molecules needed for the completely combustion of 16.55 g glucose?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:00
True or false, the three major scales used to measure earthquakes are mercalli scale, richter scale and magnitude scale
Answers: 2

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
Glucose (c6h12o6) is a key nutrient for generating chemical potential energy in biological systems....
Questions


Biology, 01.08.2019 21:30




History, 01.08.2019 21:30


Social Studies, 01.08.2019 21:30

Mathematics, 01.08.2019 21:30



Business, 01.08.2019 21:30




Social Studies, 01.08.2019 21:30

History, 01.08.2019 21:30



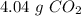
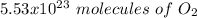
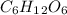 , so the first step is to find the atomic mass of each atom and multiply by the amount of atoms in the molecule.
, so the first step is to find the atomic mass of each atom and multiply by the amount of atoms in the molecule.