
The air bags in automobiles were once inflated by nitrogen gas generated by the rapid decomposition of sodium azide, nan3. if an air bag has a volume of 43.8 l and is to be filled with nitrogen gas at a pressure of 1.13 atm at a temperature of 22.4˚c, how many moles of nan3 must decompose? you may assume the n2 behaves as an ideal gas. if carmen adds zeros behind the decimal place in your answer, do not worry. it will be graded correctly.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
The air bags in automobiles were once inflated by nitrogen gas generated by the rapid decomposition...
Questions

Spanish, 25.11.2021 05:50




Mathematics, 25.11.2021 05:50





Mathematics, 25.11.2021 05:50



Mathematics, 25.11.2021 05:50


Business, 25.11.2021 05:50





Mathematics, 25.11.2021 05:50

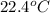 = (22.4 + 273) K = 295.4 K
= (22.4 + 273) K = 295.4 K
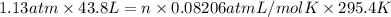
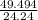
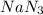 must decompose.
must decompose.

