
Chemistry, 29.11.2019 02:31 Satoetoe24
Asample of impure tin of mass 0.526 g is dissolved in strong acid to give a solution of sn2 . the solution is then titrated with a 0.0448 m solution of no3−, which is reduced to no(g). the equivalence point is reached upon the addition of 3.67×10−2 l of the no3− solution.
find the percent by mass of tin in the original sample, assuming that it contains no other reducing agents.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
Asample of impure tin of mass 0.526 g is dissolved in strong acid to give a solution of sn2 . the so...
Questions

Chemistry, 20.11.2020 05:10

Mathematics, 20.11.2020 05:10


Social Studies, 20.11.2020 05:10

Geography, 20.11.2020 05:10


Mathematics, 20.11.2020 05:10

English, 20.11.2020 05:10

History, 20.11.2020 05:10







Mathematics, 20.11.2020 05:10


Health, 20.11.2020 05:10


Mathematics, 20.11.2020 05:10

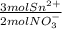 = 2.47x10⁻³mol Sn²⁺
= 2.47x10⁻³mol Sn²⁺

