Chemistry, 30.11.2019 01:31 akatsionis25
Use the following reaction: c4h9oh + nabr + h2so4 c4h9br + nahso4 + h2o if 15.0 g of c4h9oh react with 22.4 g of nabr and 32.7 g of h2so4 to yield 17.1 g of c4h9br, what is the percent yield of this reaction? remember: percent yield is your (experimental yield/theoretical yield)x100.

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3
You know the right answer?
Use the following reaction: c4h9oh + nabr + h2so4 c4h9br + nahso4 + h2o if 15.0 g of c4h9oh react w...
Questions








Mathematics, 31.03.2020 02:26







Chemistry, 31.03.2020 02:27



History, 31.03.2020 02:27


Biology, 31.03.2020 02:27

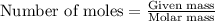 .....(1)
.....(1)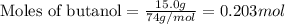
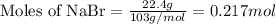
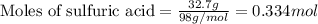
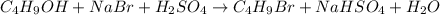
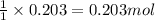 of bromobutane
of bromobutane