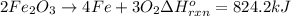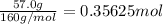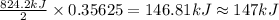Chemistry, 03.12.2019 03:31 dashavasilisk
The decomposition of 57.0 g of fe2o3 results in consider the following reaction. 2fe2o3 > 4fe + 3o2 deltah degree rxn = + 824.2 kj decomposition of 57.0 g of fe2o3 results in the release of 294 kj of heat. a. the absorption of 23500 kj of heat. b. the absorption of 147 kj of heat. c. the absorption of 294 kj of heat. d. the release of 23500 kj of heat. e. the release of 147 kj of heat.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3


Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2
You know the right answer?
The decomposition of 57.0 g of fe2o3 results in consider the following reaction. 2fe2o3 > 4fe +...
Questions

Chemistry, 20.08.2019 16:30

Mathematics, 20.08.2019 16:30

English, 20.08.2019 16:30


History, 20.08.2019 16:30




History, 20.08.2019 16:30

Mathematics, 20.08.2019 16:30


Chemistry, 20.08.2019 16:30


History, 20.08.2019 16:30


Biology, 20.08.2019 16:30



Mathematics, 20.08.2019 16:30