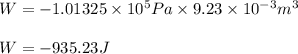Chemistry, 03.12.2019 04:31 jasmine2919
Automobile airbags contain solid sodium azide, nan 3 , that reacts to produce nitrogen gas when heated, thus inflating the bag. 2 nan 3 ( s ) ⟶ 2 na ( s ) + 3 n 2 ( g ) calculate the value of work, w , for the system if 16.5 g nan 3 reacts completely at 1.00 atm and 22 ∘ c.

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 01:00
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1

Chemistry, 23.06.2019 14:00
What mass of iron must be in a hot pack to provide -335kj of heat when the iron reacts with oxygen and is converted to iron (111) oxide according to the following thermochemical equation? 2fe(s) + 1.5o2(g)—> fe2o3(s) delta h= -824.2kj/mol
Answers: 1
You know the right answer?
Automobile airbags contain solid sodium azide, nan 3 , that reacts to produce nitrogen gas when heat...
Questions



English, 22.01.2020 04:31

History, 22.01.2020 04:31

Mathematics, 22.01.2020 04:31

Mathematics, 22.01.2020 04:31

Biology, 22.01.2020 04:31


Mathematics, 22.01.2020 04:31


Mathematics, 22.01.2020 04:31

History, 22.01.2020 04:31

Mathematics, 22.01.2020 04:31


Mathematics, 22.01.2020 04:31

Biology, 22.01.2020 04:31




Physics, 22.01.2020 04:31

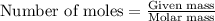
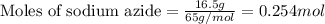
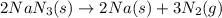
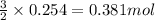 of nitrogen gas
of nitrogen gas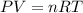
![22^oC=[22+273]K=295K](/tpl/images/0400/4731/7919f.png)
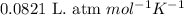
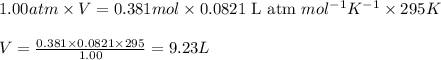
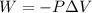
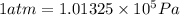 (Conversion factor: 1 atm = 101325 Pa)
(Conversion factor: 1 atm = 101325 Pa)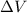 = change in volume =
= change in volume = 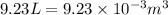 (Conversion factor:
(Conversion factor: 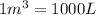 )
)