
Gaseous methane (ch4) reacts with gaseous oxygen gas (02) to produce gaseous carbon dioxide (co2) and gaseous water (h20). what is the theoretical yield of carbon dioxide formed from the reaction of 1.28 g of methane and 10.1 g of oxygen gas? be sure your answer has the correct number of significant digits in it. 02

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 01:30
List and describe the neurological effects of the vocs and other air pollutants,as described by dr.theo colborn
Answers: 2

Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
Gaseous methane (ch4) reacts with gaseous oxygen gas (02) to produce gaseous carbon dioxide (co2) an...
Questions

Mathematics, 26.09.2021 05:00

English, 26.09.2021 05:00



Mathematics, 26.09.2021 05:00


English, 26.09.2021 05:00

Computers and Technology, 26.09.2021 05:00



Chemistry, 26.09.2021 05:00


Biology, 26.09.2021 05:00


English, 26.09.2021 05:00

Chemistry, 26.09.2021 05:00


History, 26.09.2021 05:00

Chemistry, 26.09.2021 05:00

Mathematics, 26.09.2021 05:00

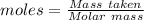
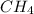 :-
:- 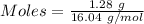
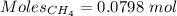
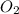 :-
:-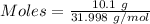
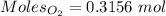
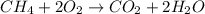
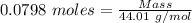
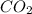 = 3.51 g
= 3.51 g

