
Chemistry, 05.12.2019 05:31 Jcausey4477
A) how many moles of co2 and h2o are formed from 3.85 mole of propane c3h8 (this calculation needs to be done twice-once fro co2 and once for h20.
b) if 0.647 mole of oxygen in used in the burning of propane, how many moles of each of h2o are produced? how many moles of c3h8 are consumed?
the balanced chemical reaction:
1 c3 + 5 o2 = 3co2 + 4 h2o

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

You know the right answer?
A) how many moles of co2 and h2o are formed from 3.85 mole of propane c3h8 (this calculation needs t...
Questions












English, 16.01.2020 04:31



Social Studies, 16.01.2020 04:31





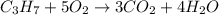
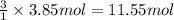 of carbon dioxide gas.
of carbon dioxide gas.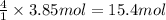 of water .
of water .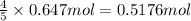 of water.
of water.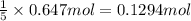 of propane.
of propane.

