
Chemistry, 18.12.2019 03:31 dalechloe5596
Which of the following processes would you predict to have an increase in entropy?
condensation of water
freezing water
melting ice
deposition of co2 (changing from a gas to solid)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 14:30
Which statement best identifies the process shown? the process must be fusion because energy is released. a.the process must be fusion because a heavier nucleus forms from smaller nuclei. b.the process must be fission because a large nucleus breaks into smaller nuclei. c.the process must be fission because neutrons are formed.
Answers: 1
You know the right answer?
Which of the following processes would you predict to have an increase in entropy?
cond...
cond...
Questions

Mathematics, 08.07.2019 12:00

Mathematics, 08.07.2019 12:00




Mathematics, 08.07.2019 12:00







History, 08.07.2019 12:00

History, 08.07.2019 12:00

History, 08.07.2019 12:00

Social Studies, 08.07.2019 12:00


History, 08.07.2019 12:00


History, 08.07.2019 12:00

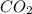 : deposition process where gaseous state changes to solid state by escaping liquid state, thus decreasing randomness and decreasing entropy.
: deposition process where gaseous state changes to solid state by escaping liquid state, thus decreasing randomness and decreasing entropy.

