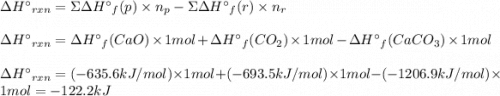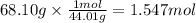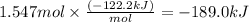Chemistry, 05.12.2019 22:31 20stirltrer
At 850°c, caco3 undergoes substantial decomposition to yield cao and co2. assuming that the δh o f values of the reactant and products are the same at 850°c as they are at 25°c, calculate the enthalpy change (in kj) if 68.10 g of co2 is produced in one reaction.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

You know the right answer?
At 850°c, caco3 undergoes substantial decomposition to yield cao and co2. assuming that the δh o f v...
Questions


English, 24.09.2020 03:01





Physics, 24.09.2020 03:01

Mathematics, 24.09.2020 03:01

History, 24.09.2020 03:01

Computers and Technology, 24.09.2020 03:01




Mathematics, 24.09.2020 03:01



Mathematics, 24.09.2020 03:01

Mathematics, 24.09.2020 03:01

Mathematics, 24.09.2020 03:01

Mathematics, 24.09.2020 03:01