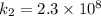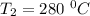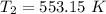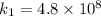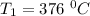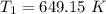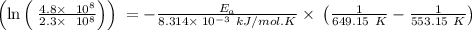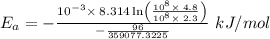Chemistry, 06.12.2019 02:31 jessicamcgoldri5625
The rate constant k for a certain reaction is measured at two different temperatures temperature 376.0 °c 4.8 x 108 280.0 °c 2.3 x 10 8 assuming the rate constant obeys the arrhenius equation, calculate the activation energy e for this reaction. round your answer to 2 significant digits. kj x10 mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

You know the right answer?
The rate constant k for a certain reaction is measured at two different temperatures temperature 376...
Questions

Mathematics, 13.05.2021 01:00



Mathematics, 13.05.2021 01:00


Mathematics, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00

Biology, 13.05.2021 01:00



Mathematics, 13.05.2021 01:00





Mathematics, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00

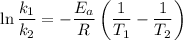
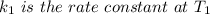
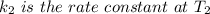
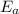 is the activation energy
is the activation energy