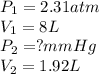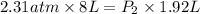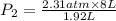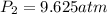a volume of 8 l. if the gas is compressed to


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2


Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Agas has a pressure of 2.31 atm and occupies
a volume of 8 l. if the gas is compressed to
a volume of 8 l. if the gas is compressed to
Questions







Mathematics, 19.12.2019 06:31


Law, 19.12.2019 06:31


History, 19.12.2019 06:31

Advanced Placement (AP), 19.12.2019 06:31

Mathematics, 19.12.2019 06:31

Physics, 19.12.2019 06:31

Mathematics, 19.12.2019 06:31


History, 19.12.2019 06:31

Mathematics, 19.12.2019 06:31


History, 19.12.2019 06:31

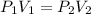 (At constant temperature)
(At constant temperature)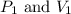 are initial pressure and volume.
are initial pressure and volume.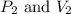 are final pressure and volume.
are final pressure and volume.