
Chemistry, 06.12.2019 05:31 AaronMicrosoft15
If the equilibrium constant for a two-electron redox reaction at 298 k is 1.0×10−4, calculate the corresponding δg∘ and e∘cel under standard conditions.
δg∘ =
e∘cell =

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2
You know the right answer?
If the equilibrium constant for a two-electron redox reaction at 298 k is 1.0×10−4, calculate the co...
Questions

Mathematics, 09.03.2021 06:00

Mathematics, 09.03.2021 06:00



Mathematics, 09.03.2021 06:00

Mathematics, 09.03.2021 06:00

English, 09.03.2021 06:00


Arts, 09.03.2021 06:00

Social Studies, 09.03.2021 06:00






Mathematics, 09.03.2021 06:00


Geography, 09.03.2021 06:00


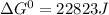
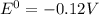
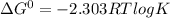
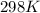
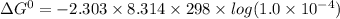
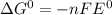
 = gibbs free energy = 22823J
= gibbs free energy = 22823J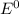 = standard emf
= standard emf



