
Chemistry, 06.12.2019 19:31 alyssalefeber
The molal boiling point elevation constant kb= 2.13 ℃kgmo-for a certain substance x, when 12. g of urea are dissolved in 100. g of x, the solution boils at 126.3 °c. calculate the boiling point of pure x. be sure your answer has the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

You know the right answer?
The molal boiling point elevation constant kb= 2.13 ℃kgmo-for a certain substance x, when 12. g of u...
Questions









History, 13.07.2019 18:00


History, 13.07.2019 18:00


Mathematics, 13.07.2019 18:00


English, 13.07.2019 18:00

Mathematics, 13.07.2019 18:00

Health, 13.07.2019 18:00

English, 13.07.2019 18:00

Mathematics, 13.07.2019 18:00

English, 13.07.2019 18:00

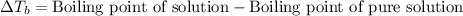
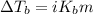
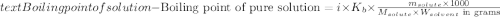
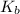 = molal boiling point elevation constant = 2.13°C/m
= molal boiling point elevation constant = 2.13°C/m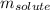 = Given mass of solute (urea) = 12. g
= Given mass of solute (urea) = 12. g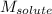 = Molar mass of solute (urea) = 60 g/mol
= Molar mass of solute (urea) = 60 g/mol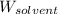 = Mass of solvent (X) = 100. g
= Mass of solvent (X) = 100. g


