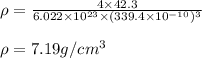Chemistry, 22.08.2019 08:30 domenica19
The atomic radius of metal x is 1.20 × 102 picometers (pm) and a crystal of metal x has a unit cell that is face-centered cubic. calculate the density of metal x? (atomic weight = 42.3 g/mol)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 23.06.2019 06:00
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
You know the right answer?
The atomic radius of metal x is 1.20 × 102 picometers (pm) and a crystal of metal x has a unit cell...
Questions


Mathematics, 15.07.2019 15:00

English, 15.07.2019 15:00



Computers and Technology, 15.07.2019 15:00

Mathematics, 15.07.2019 15:00



Mathematics, 15.07.2019 15:00

Mathematics, 15.07.2019 15:00


English, 15.07.2019 15:00


English, 15.07.2019 15:00

Mathematics, 15.07.2019 15:00

History, 15.07.2019 15:00

Mathematics, 15.07.2019 15:00


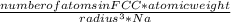 . Substituting the given, the density is 162.69 g/cm3.
. Substituting the given, the density is 162.69 g/cm3. 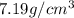
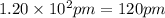
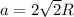
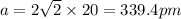
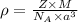
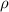 = density
= density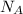 = Avogadro's number =
= Avogadro's number = 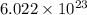
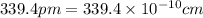 (Conversion factor:
(Conversion factor: 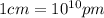 )
)