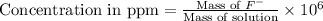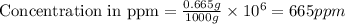Chemistry, 09.12.2019 23:31 camiloriveraveoxbgd6
Express the concentration of a 0.0350 m aqueous solution of fluoride, f − , in mass percentage and in parts per million (ppm). assume the density of the solution is 1.00 g/ml. a. number in percentage %
b. number in ppm .

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
Express the concentration of a 0.0350 m aqueous solution of fluoride, f − , in mass percentage and i...
Questions





Mathematics, 21.09.2019 16:10

Physics, 21.09.2019 16:10

Biology, 21.09.2019 16:10

Mathematics, 21.09.2019 16:10

Biology, 21.09.2019 16:10

Mathematics, 21.09.2019 16:10

Biology, 21.09.2019 16:10

Health, 21.09.2019 16:10


English, 21.09.2019 16:10


Health, 21.09.2019 16:10



Mathematics, 21.09.2019 16:10

Mathematics, 21.09.2019 16:10

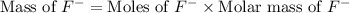
 = 19 g/mole
= 19 g/mole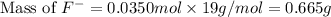
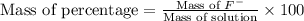
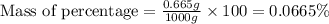
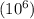 parts by the mass of the solution.
parts by the mass of the solution.