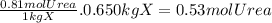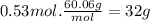Chemistry, 10.12.2019 01:31 Leanylopez0811
Acertain substance x has a normal boiling point of 124.2 °c and a molal boiling point elevation constant k,-06-0c-kg-mol ·a solution is prepared by dissolving some urea ((n112)co) in 650. g ofl. this solution boils at 124.7 oc, calculate the mass of urea that was dissolved. be sure your answer has the correct number of significant digits. 31.48 g

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 23.06.2019 05:30
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3

Chemistry, 23.06.2019 07:00
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table?
Answers: 2

Chemistry, 23.06.2019 09:30
Where are the noble gases located in the periodic table? a. in the center b. on the left side c. in the upper right corner d. on the far right side
Answers: 1
You know the right answer?
Acertain substance x has a normal boiling point of 124.2 °c and a molal boiling point elevation cons...
Questions

English, 30.07.2019 07:00


English, 30.07.2019 07:00

Physics, 30.07.2019 07:00

Biology, 30.07.2019 07:00

English, 30.07.2019 07:00

Biology, 30.07.2019 07:00


Biology, 30.07.2019 07:00




History, 30.07.2019 07:00



Mathematics, 30.07.2019 07:00

Mathematics, 30.07.2019 07:00


History, 30.07.2019 07:00