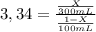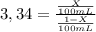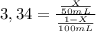Chemistry, 10.12.2019 04:31 lilianaalbarrap9tkqo
Achemist wants to extract a solute from 100 ml of water using only 300 ml of ether. the partition coefficient between ether and water is 3.34. calculate the fraction of solute that would remain in the water under each of the extraction conditions.
a. the chemist performs a single extraction with 300 ml of ether.
b. the chemist performs three extractions with 100 ml of ether.
c. the chemist performs six extractions with 50 ml of ether.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

You know the right answer?
Achemist wants to extract a solute from 100 ml of water using only 300 ml of ether. the partition co...
Questions


Mathematics, 27.11.2020 01:50

Chemistry, 27.11.2020 01:50

Biology, 27.11.2020 01:50

Mathematics, 27.11.2020 01:50

History, 27.11.2020 01:50