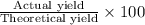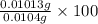Chemistry, 11.12.2019 04:31 jessicalivchits8486
Avolume of 129 ml of hydrogen is collected over water. the water level in the collecting vessel is the same as the outside level. atmospheric pressure is 756.0 torr and the temperature is 25 ∘ c. what is the percent yield of hydrogen for this reaction?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
Avolume of 129 ml of hydrogen is collected over water. the water level in the collecting vessel is t...
Questions


Biology, 05.11.2020 01:30


Mathematics, 05.11.2020 01:30


Arts, 05.11.2020 01:30


Mathematics, 05.11.2020 01:30


Mathematics, 05.11.2020 01:30


English, 05.11.2020 01:30

Mathematics, 05.11.2020 01:30

Social Studies, 05.11.2020 01:30





English, 05.11.2020 01:30

Mathematics, 05.11.2020 01:30

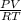
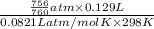
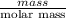
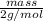
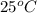
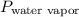 = 24 mm Hg
= 24 mm Hg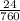 atm
atm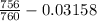
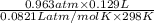
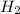 = 0.0056 mol × 2
= 0.0056 mol × 2