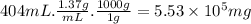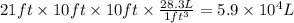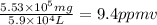Chemistry, 11.12.2019 18:31 serenityarts123
Aclumsy chemist drops a beaker containing 404 ml of a solvent (mw = 88.2, sg = 1.37) in a room measuring 21 ft x 10 ft x 10 ft. assuming that the contents of the beaker completely evaporate and fill the space, what is the resulting concentration in parts per million by volume (ppmv)? assume normal temperature and pressure (ntp), i. e., p = 1 atm, and t = 25 celsius. 1 ft3 = 28.3 l

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

You know the right answer?
Aclumsy chemist drops a beaker containing 404 ml of a solvent (mw = 88.2, sg = 1.37) in a room measu...
Questions

Chemistry, 08.10.2019 06:10


Mathematics, 08.10.2019 06:10


Mathematics, 08.10.2019 06:10


Mathematics, 08.10.2019 06:10

Mathematics, 08.10.2019 06:10

Mathematics, 08.10.2019 06:10

Mathematics, 08.10.2019 06:10

Mathematics, 08.10.2019 06:10


Mathematics, 08.10.2019 06:10



Mathematics, 08.10.2019 06:10


Mathematics, 08.10.2019 06:10

Physics, 08.10.2019 06:10