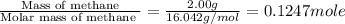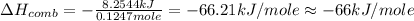Chemistry, 11.12.2019 19:31 wcraig1998
When 2.00 g of methane are burned in a bomb calorimeter, the change in temperature is 3.08°c. the heat capacity of the calorimeter is 2.68 kj/°c. the molar mass of methane is 16.042 g/mol. what is the approximate molar enthalpy of combustion of this substance?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Imagine that you own a property that is exactly 2.2 acres large. you want to sell your property, but your realtor tells you that you cannot sell your land by the acre. in order to sell your land you need to determine the area you own in units of square meters? given that there are 1.6 kilometers in 1 mile and 640 acres in 1 square mile, what is the area of land that you own in square meters square meters?
Answers: 2

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
You know the right answer?
When 2.00 g of methane are burned in a bomb calorimeter, the change in temperature is 3.08°c. the he...
Questions

Biology, 14.05.2021 04:40


Mathematics, 14.05.2021 04:40


History, 14.05.2021 04:40


Physics, 14.05.2021 04:40

Mathematics, 14.05.2021 04:40

Computers and Technology, 14.05.2021 04:40


Mathematics, 14.05.2021 04:40









Mathematics, 14.05.2021 04:40

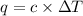
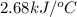
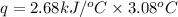
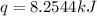
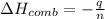
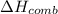 = enthalpy change = ?
= enthalpy change = ?