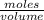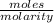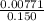Chemistry, 11.12.2019 22:31 ginger87771
What volume (in ml) of a 0.150 m hno 3 solution will completely react with 35.7 ml of a 0.108 m na 2 co 3 solution according to this balanced chemical equation?
na 2 co 3 ( a q ) + 2 hno 3 ( a q ) → 2 nano 3 ( a q ) + co 2 ( g ) + h 2 o ( l )

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 23.06.2019 07:20
Which of the following are acids or bases? 1. sodium hydrogen 2. barium hydroxide solution 3. carbonate solution
Answers: 1

Chemistry, 23.06.2019 11:00
Which of the following reactions represents an exothermic reaction? nh3(g) + 12.0 kcal ½n2(g) + 3/2 h2(g) ch4 + 2o2 co2 + 2h2o + 212,800 cal c + 2s cs2, h = 27,550 cal c(graphite) c(diamond), h = 0.45 kcal 2h2o 2h2 + o2, h = +58 kcal
Answers: 1

Chemistry, 23.06.2019 13:00
Johnny's bakery has 30,900 grams of sugar. a recipe calls for 32 pounds of sugar to be used. how much sugar will be left over? (1 lb=453.59 g).
Answers: 2
You know the right answer?
What volume (in ml) of a 0.150 m hno 3 solution will completely react with 35.7 ml of a 0.108 m na 2...
Questions


Chemistry, 14.07.2019 23:00





Business, 14.07.2019 23:00


Mathematics, 14.07.2019 23:00

Chemistry, 14.07.2019 23:00


Mathematics, 14.07.2019 23:00




Physics, 14.07.2019 23:00


Mathematics, 14.07.2019 23:00