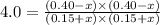Chemistry, 12.12.2019 03:31 antoniaannswiney
Acetic acid, ch3co2h, reacts with ethanol, c2h5oh, to form water and ethyl acetate, ch3co2c2h5. the equilibrium constant for this reaction with dioxane as a solvent is 4.0. what are the equilibrium concentrations for a mixture that is initially 0.15 m in ch3co2h, 0.15 m in c2h5oh, 0.40 m in ch3co2c2h5, and 0.40 m in h2o?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3


Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
Acetic acid, ch3co2h, reacts with ethanol, c2h5oh, to form water and ethyl acetate, ch3co2c2h5. the...
Questions


Mathematics, 09.02.2021 21:00

Social Studies, 09.02.2021 21:00

English, 09.02.2021 21:00

Mathematics, 09.02.2021 21:00



Mathematics, 09.02.2021 21:00

World Languages, 09.02.2021 21:00

Mathematics, 09.02.2021 21:00

Computers and Technology, 09.02.2021 21:00

Mathematics, 09.02.2021 21:00

History, 09.02.2021 21:00


Mathematics, 09.02.2021 21:00



Mathematics, 09.02.2021 21:00


English, 09.02.2021 21:00

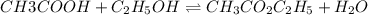
![K_c=\frac{[CH_3CO_2C_2H_5][H_2O]}{[CH_3COOH][C_2H_5OH]}](/tpl/images/0414/6294/2612b.png)