Chemistry, 14.12.2019 08:31 Nathaliasmiles
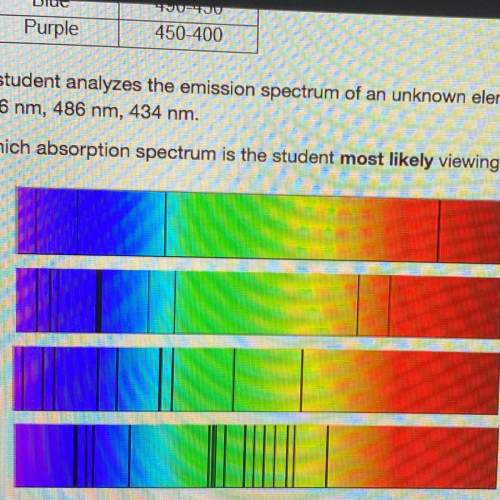
Astudent analyzes the emission spectrum of an unknown element and observes strong lines at the following wavelengths:
656 nm, 486 nm, 434 nm.
which absorption spectrum is the student most likely viewing?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1
You know the right answer?
Astudent analyzes the emission spectrum of an unknown element and observes strong lines at the follo...
Questions


English, 11.04.2021 01:20

Arts, 11.04.2021 01:20



Mathematics, 11.04.2021 01:20


Mathematics, 11.04.2021 01:20


Social Studies, 11.04.2021 01:20

Mathematics, 11.04.2021 01:20

Social Studies, 11.04.2021 01:20


Arts, 11.04.2021 01:20


Arts, 11.04.2021 01:20




Mathematics, 11.04.2021 01:20