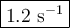Chemistry, 16.12.2019 22:31 caveman171
If the activation energy for a given compound is found to be 103 kj/mol, with a frequency factor of 4.0 × 1013 s-1, what is the rate constant for this reaction at 398 k? 2.5 × 107 s-18.2 s-13.9 × 1010 s-11.2 s-11.7 × 1010 s-1

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
If the activation energy for a given compound is found to be 103 kj/mol, with a frequency factor of...
Questions

Mathematics, 23.02.2020 21:01


Biology, 23.02.2020 21:01


Mathematics, 23.02.2020 21:02

Mathematics, 23.02.2020 21:02

Business, 23.02.2020 21:02


Mathematics, 23.02.2020 21:02

Physics, 23.02.2020 21:03


Mathematics, 23.02.2020 21:03

Mathematics, 23.02.2020 21:03

Mathematics, 23.02.2020 21:03

History, 23.02.2020 21:03

Mathematics, 23.02.2020 21:04

Health, 23.02.2020 21:04

History, 23.02.2020 21:04


Mathematics, 23.02.2020 21:05