Chemistry, 17.12.2019 04:31 coolquezzie
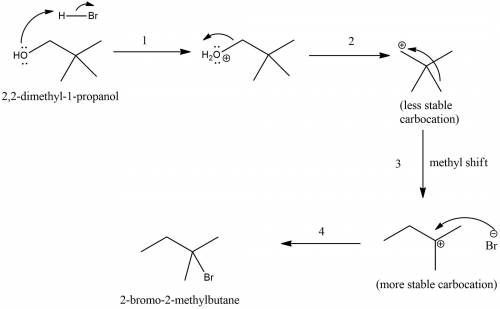
The reaction of 2,2-dimethyl-1-piopanol with hbr is very slow and gives 2-bromo- 2-methyibutane as the major product. give a mechanistic explanation for these observations. select all that apply. stereoelectronic effects result in an antic op lanar rearrangement of the carbon skeleton. steric hindrance prevents nucleophilic attack. the mechanism requites the development of an unstable positively charged species in the transition state. the mechanism results in a carbocation rearrangement in which a methyl shift occurs. the mechanism requires dissociation of a poor leaving group.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
You know the right answer?
The reaction of 2,2-dimethyl-1-piopanol with hbr is very slow and gives 2-bromo- 2-methyibutane as t...
Questions


Social Studies, 07.07.2020 03:01

Mathematics, 07.07.2020 03:01


Mathematics, 07.07.2020 03:01

Mathematics, 07.07.2020 03:01






Mathematics, 07.07.2020 03:01

Chemistry, 07.07.2020 03:01

Mathematics, 07.07.2020 03:01

Mathematics, 07.07.2020 03:01





Chemistry, 07.07.2020 03:01

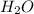 .
.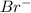 attacks the stable carbocation to produce 2-bromo-2-methylbutane.
attacks the stable carbocation to produce 2-bromo-2-methylbutane.