

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:30
What would most likely be the transmittance of a 0.70 m solution of solute a?
Answers: 1

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1
You know the right answer?
If solid nacl is added to a saturated water solution of pbcl2 at 20o c, a precipitate is formed. how...
Questions














Computers and Technology, 01.11.2019 05:31






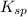 remains the same
remains the same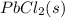 ⇄
⇄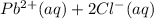
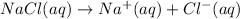
![K_{sp}=[Pb^{2+}][Cl^-]^2](/tpl/images/0424/5297/7fd11.png)
![S=[Pb^{2+}]=\frac{K_{sp}}{[Cl^-]^2}](/tpl/images/0424/5297/c2c31.png)



