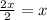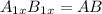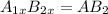In a hexagonal-close-pack (hcp) unit cell, the ratio of lattice points to octahedral holes to tetrahedral holes is 1: 1: 2. what is the general formula for a compound if anions occupy the hcp lattice points and cations occupy half of the tetrahedral holes? ab a3b ab2 a2b what is the general formula for a compound if anions occupy the hcp lattice points and cations occupy all the tetrahedral holes? ab a2b ab2 a3b

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
In a hexagonal-close-pack (hcp) unit cell, the ratio of lattice points to octahedral holes to tetrah...
Questions

Biology, 19.01.2021 16:50


Mathematics, 19.01.2021 16:50

Mathematics, 19.01.2021 16:50

Mathematics, 19.01.2021 16:50





Mathematics, 19.01.2021 16:50



Law, 19.01.2021 16:50


English, 19.01.2021 16:50


Mathematics, 19.01.2021 16:50


History, 19.01.2021 16:50

Mathematics, 19.01.2021 16:50